Cholera infections, caused by Vibrio cholerae bacteria, are known to be life-threatening due to the production of the cholera toxin. This toxin binds to specific “sugar lipids” (GM1 gangliosides) on intestinal cell surfaces, creating one of the strongest interactions between a protein and sugar molecules. This bond allows the toxin to enter intestinal cells, leading to rapid fluid loss.
In a collaborative effort involving researchers from the University of Münster, ETH Zürich, and Leibniz Universität Hannover, a key component of the GM1 cholera toxin complex was studied utilizing a fluorinated GM1 analog. By examining the molecular mechanisms behind this strong interaction, the study aimed to provide insights into the disease and potentially facilitate the development of new medications.
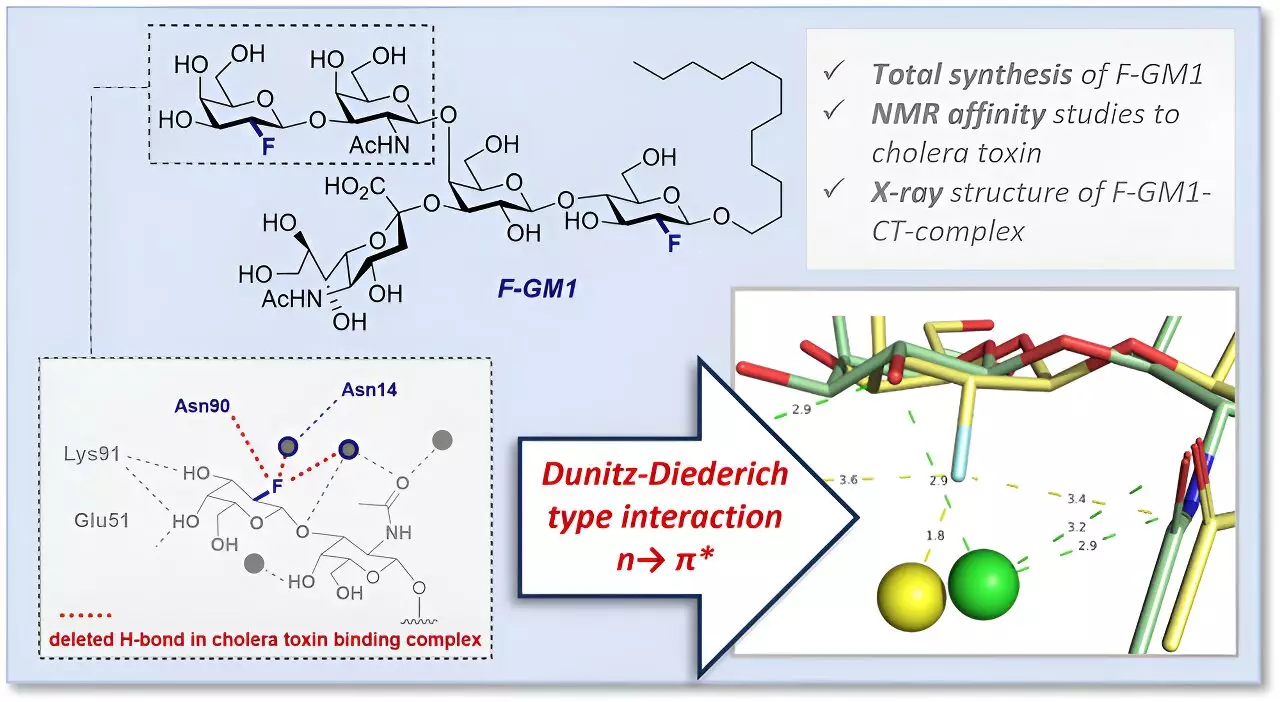
The fluorinated GM1 analog (F-GM1) synthesized by the research team played a crucial role in elucidating the molecular interactions between GM1 gangliosides and the cholera toxin. Fluorine served as a valuable tool, offering benefits such as enhanced molecule stability, precise control of atom arrangement, and the ability to investigate interactions using nuclear magnetic resonance spectroscopy.
By utilizing protein crystallography, the researchers were able to delve deeper into the interactions and spatial configuration of F-GM1 within the binding pocket. This analysis revealed additional interactions facilitated by fluorine, as well as alterations in the arrangement of functional groups within the binding site. These findings shed light on the slightly lower affinity of the analog for cholera toxin compared to natural GM1.
The study underscores the potential of employing fluorinated gangliosides in biomedical investigations. Beyond studying molecular signaling pathways involving sugar chains, known as glycans, these compounds could be instrumental in identifying novel drug candidates and advancing vaccine development efforts. The findings pave the way for further exploration of fluorine-based analogs in various research areas.


Leave a Reply