Ice has long been considered a key player in the development of life on Earth. The way in which organic molecules can be trapped within ice crystal lattice structures has led scientists to believe that concentration of these compounds could have contributed to the emergence of life. However, traditional methods of studying organic molecules in ice, such as Raman and infrared spectroscopy, have limitations when it comes to measurement sensitivity.
A team of researchers from the University of Science and Technology of China (USTC) led by Prof. Zhang Guoqing, Prof. Liu Shiyong, Prof. Zhou Xiaoguo, and Researcher Zhang Xuepeng, have developed an innovative method for detecting microstructures in water ice using organic phosphorescent probes and phosphorescence spectroscopy. This method is a departure from traditional absorption-based spectroscopic techniques, offering a new way to study organic molecules in ice more effectively.
The team’s approach involves using a phosphorescent probe called acridinium iodide (ADI) to indicate changes in the microstructural composition of water ice. By observing the hydration state of the ADI probe, researchers were able to differentiate between crystalline and glassy forms of water ice. In amorphous ice, the ADI probe exhibits long-lasting phosphorescence with a greenish-yellow afterglow, while in crystalline ice, the probe induces short-lived red phosphorescence due to molecular aggregation.
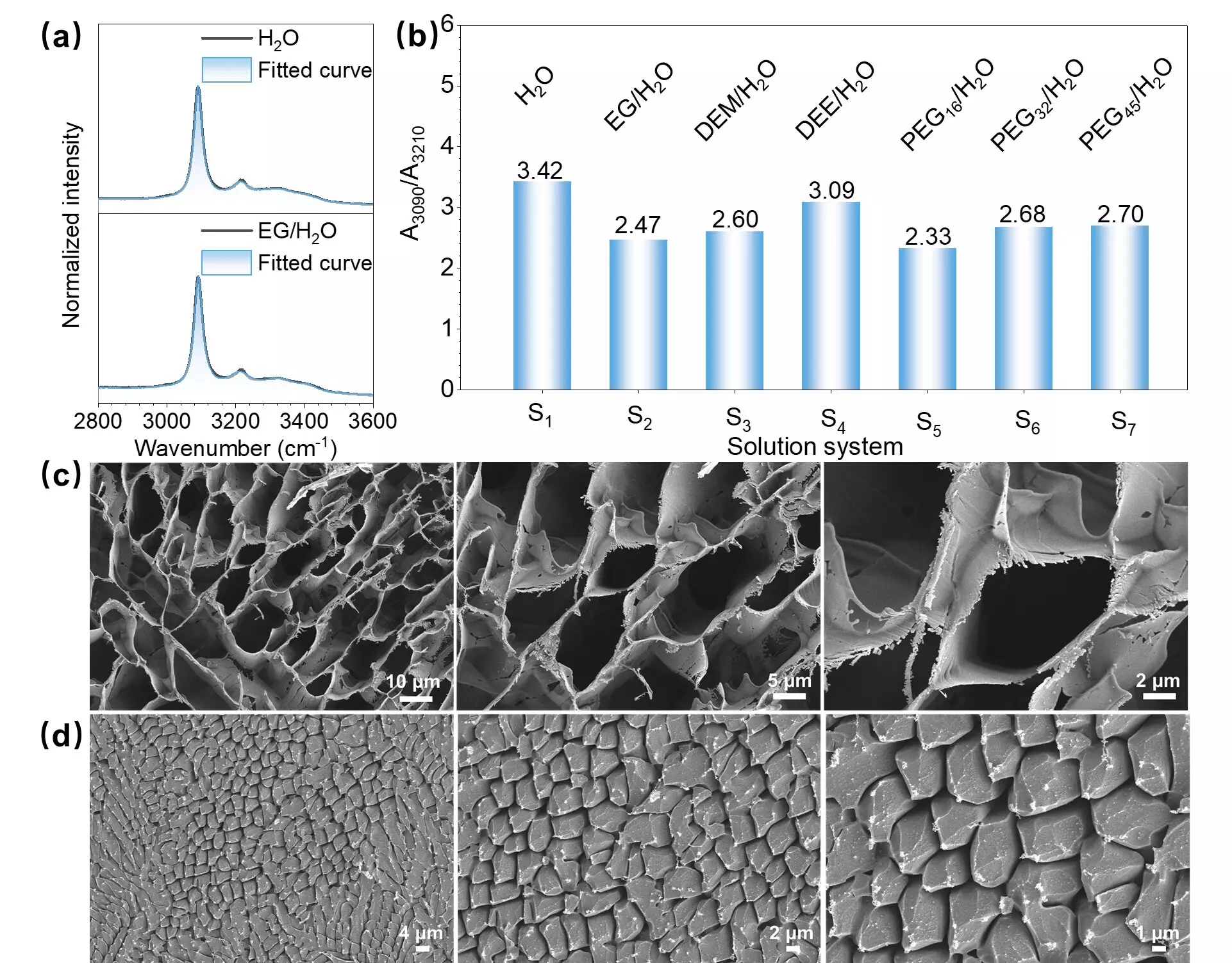
Through emission spectra analysis, the researchers were able to observe distinct changes in the behavior of ADI molecules when small amounts of ethylene glycol (EG) were added to the water ice. The addition of EG led to a transformation in the state of ADI molecules from undissolved aggregates to dissolved ion states, as evidenced by shifts in fluorescence and phosphorescence bands in the spectra.
To validate their findings, the researchers utilized low-temperature scanning electron microscopy (Cryo-SEM) images and low-temperature Raman (LT-Raman) spectra. These additional analyses confirmed the impact of trace amounts of EG on the microstructures of water ice, corroborating the conclusions drawn from phosphorescence spectroscopy. The images showed local areas with porous microstructures when EG was added, while the Raman spectra displayed shifts in the O-H vibration of water ice, indicating changes from a crystalline to a glassy state.
This study has provided valuable insights into the interaction between water, ice, and organic molecules. By demonstrating the inhibitory effect of organic molecules on the crystalline order of water ice, the researchers have opened up new possibilities for studying these interactions at lower concentrations and across a wider temperature range. The use of phosphorescence spectroscopy offers a more convenient and sensitive method for investigating water-ice-organics interactions, providing a new avenue for scientific exploration in this area.

Leave a Reply