For over 200 years, scientists have been grappling with a perplexing geology mystery known as the “Dolomite Problem.” Dolomite, a common mineral found in various geological formations, has been notoriously difficult to grow in laboratory settings under conditions believed to mimic its natural formation. However, a breakthrough study conducted by researchers from the University of Michigan and Hokkaido University in Sapporo, Japan has finally provided an answer to this long-standing conundrum.
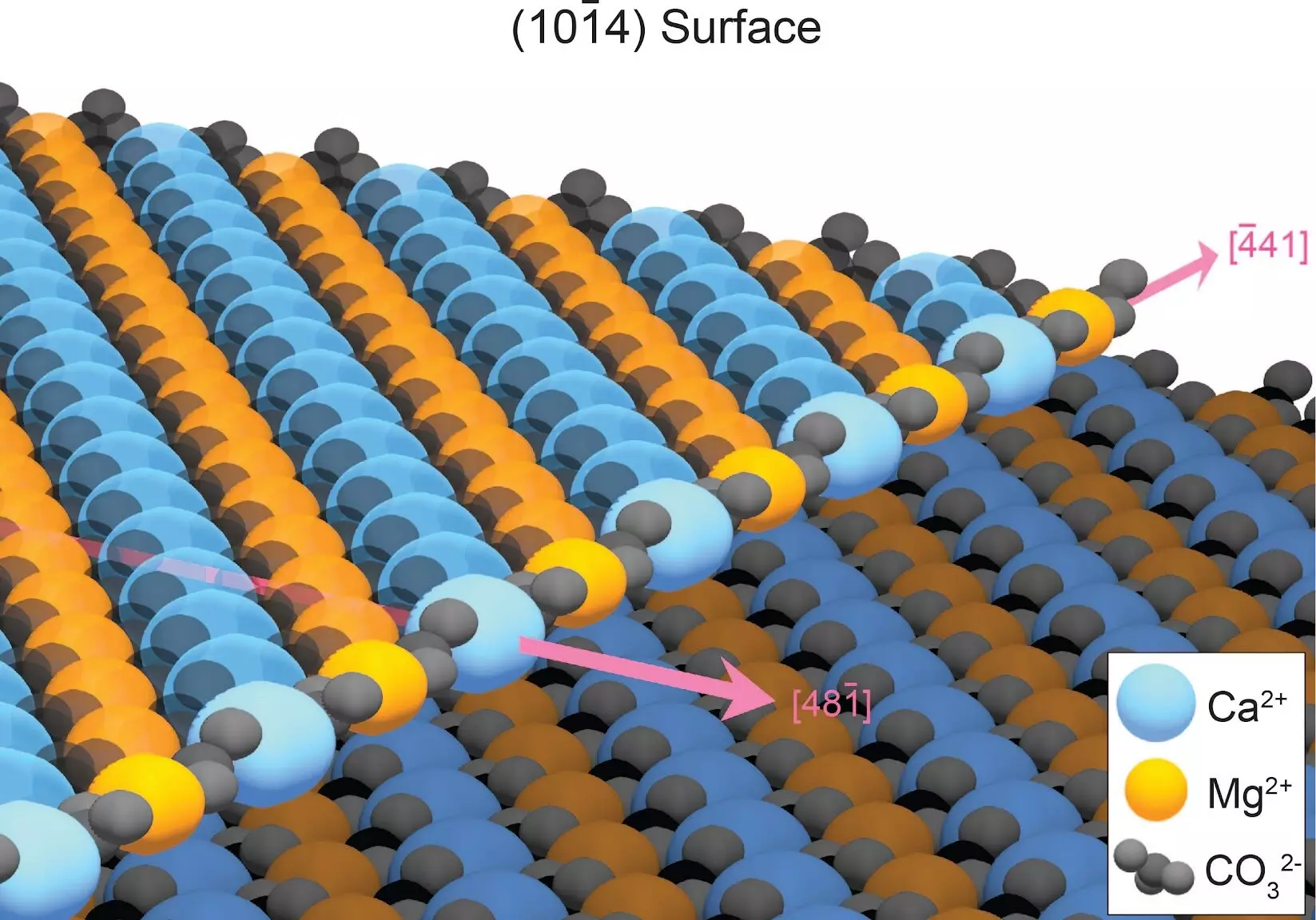
The key to successfully growing dolomite in the lab lies in understanding how defects in the mineral structure hinder its formation. When minerals crystallize in water, atoms typically adhere to the crystal surface in an orderly manner. However, dolomite presents a unique challenge as its growth edge consists of alternating rows of calcium and magnesium. As a result, calcium and magnesium atoms tend to attach randomly, creating defects that impede the growth of additional dolomite layers. This disorder greatly slows down dolomite growth, with estimates suggesting it would take 10 million years to form just one layer of ordered dolomite.
Fortunately, these defects can be overcome. The disordered atoms are less stable than those in their correct positions, making them more susceptible to dissolution. By repeatedly rinsing away these defects, such as through rain or tidal cycles, dolomite layers can form in a matter of years, eventually leading to the accumulation of dolomite mountains over geological time.
To accurately simulate dolomite growth, the researchers developed a computational model that calculates the energy associated with the atomic interactions occurring during the crystal formation process. This approach allows for the prediction of how strongly or weakly atoms will attach to an existing dolomite surface. While these simulations typically demand significant computing power, the researchers made use of software developed at the University of Michigan’s Predictive Structure Materials Science (PRISMS) Center, which provided a shortcut by extrapolating energies based on the crystal’s symmetry. This advancement enabled them to simulate dolomite growth over geologic timescales in a fraction of the time previously required.
To validate their theory, the researchers turned to transmission electron microscopes and a unique property of electron beams. By pulsing the beam multiple times, they were able to dissolve away defects in tiny dolomite crystals immersed in a solution of calcium and magnesium. After the pulses, dolomite growth was observed, with approximately 100 nanometers (around 250,000 times smaller than an inch) of dolomite forming – an unprecedented achievement considering that previous attempts in the lab had only managed to grow a maximum of five layers. This experimental confirmation provided concrete evidence to support the researchers’ findings.
The knowledge gained from solving the Dolomite Problem holds significant implications beyond the field of geology. This breakthrough can inform the development of strategies for promoting the growth of technologically important materials. By understanding and mitigating crystal defects, engineers can manufacture higher-quality materials for various applications, including semiconductors, solar panels, batteries, and other technologies.
The Dolomite Problem also challenges the traditional approach to crystal growth. In the past, researchers seeking defect-free materials would attempt to grow them slowly to reduce the occurrence of defects. However, this study demonstrates that defects can be effectively removed through methods such as repeated rinsing or dissolution, offering a new avenue for creating defect-free materials in a more time-efficient manner.
The successful growth of dolomite in laboratory conditions, resolving the long-standing Dolomite Problem, marks a significant milestone in geology research. It not only sheds light on the formation of this abundant mineral in nature but also has broader implications for materials science and engineering. By understanding and harnessing the mechanisms behind crystal growth, scientists and engineers can unlock new possibilities and develop better materials for a wide range of applications.

Leave a Reply