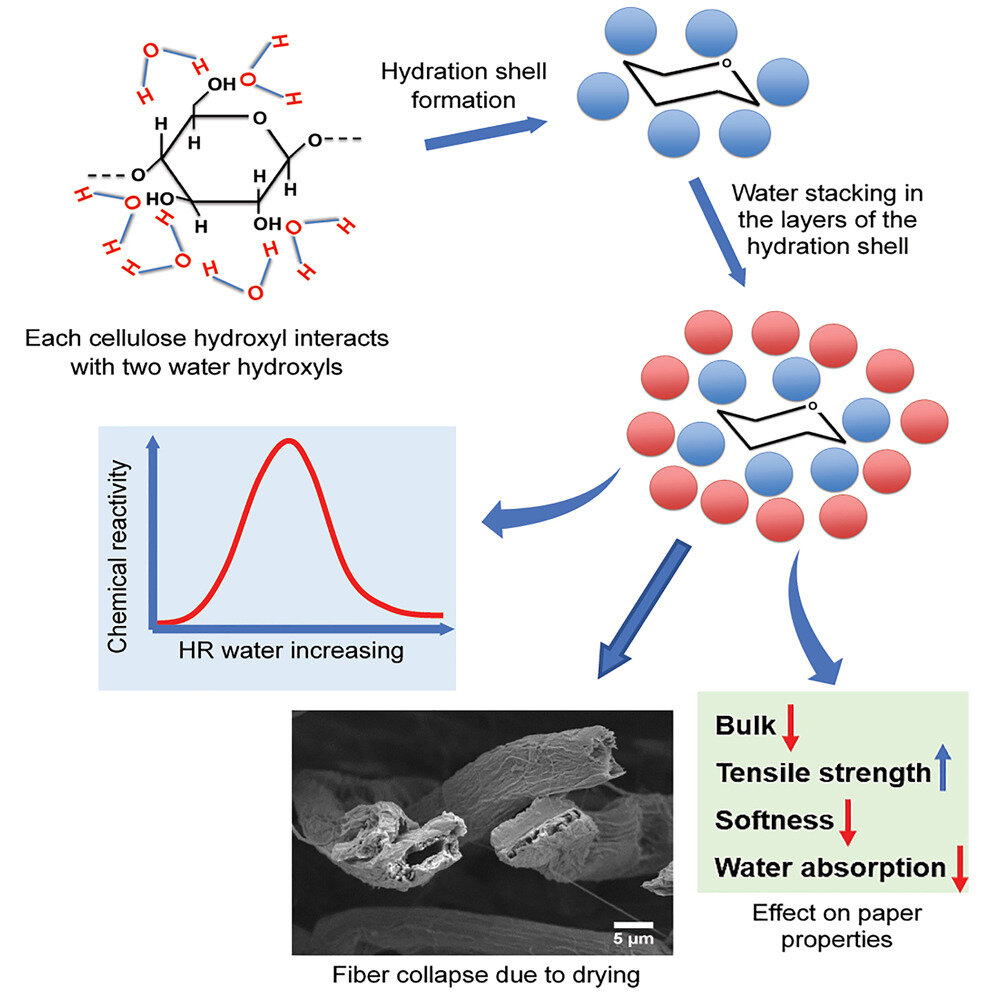
Water is known as a universal solvent that remains unchanged by its interactions. However, recent research from North Carolina State University has shown that water can change its solubility characteristics, depending on what it interacts with. This research has found that when water interacts with cellulose, it can stack in layered shells, controlling chemical reactions within the material.
According to Lucian Lucia, Professor of Forest Biomaterials and Chemistry at NC State, cellulose is the world’s most abundant biopolymer, and it is used in a variety of applications. These applications range from bandages to electronics. However, cellulose processing has been done by trial and error, and some of it utilizes harsh chemicals. To find better ways to process cellulose, scientists need to understand its most fundamental interactions, such as the interaction with water.
Jim Martin, Professor of Chemistry at NC State, who studies the fundamental properties of water as a solvent, explains that water has the ability to change its characteristics depending on what it is with. This gives it a wide range of solubility characteristics. “We change the nature of water by what we dissolve in it, and by the concentrations of those solutes in water,” Martin says.
Lucia and Martin physically manipulated different types of wood fibers to see how water bound to itself and other molecules within the resulting structures. They saw that at lower water contents, the water distribution and resulting molecular interactions between the water and the fibers create bridging structures within the material, causing it to lose flexibility. Additionally, the water can “hide” itself within the cellulose network, forming strong hydrogen bonds. This bonding dictates the tightness or looseness of the bridging structures. “The water forms shells around the fibers that can stack, like a nesting Russian doll,” Martin says.
The researchers hope to explore the variety of bonds water forms within these structures in future work. This research has significant implications for the more sustainable and efficient design of cellulose-based products. By understanding the fundamental interactions of cellulosic materials and water, scientists may be able to develop more efficient and less harsh ways to process cellulose.
Despite the term "rare," rare earth metals (REMs) are not nearly as scarce as their…
A collaboration led by Rutgers University-New Brunswick has initiated a paradigm shift in our understanding…
Natural gas leaks are a growing concern in both urban and rural settings, with potential…
Recent groundbreaking research at the University of Vienna has unveiled a novel interplay of forces…
In recent years, perovskites have garnered significant attention in the fields of materials science and…
For decades, astronomers have probed the depths of the Milky Way, grappling with two perplexing…
This website uses cookies.