Carbohydrates, known as sugar chains or “glycans” in the body, play a crucial role in various biological processes by attaching themselves to proteins and lipids. However, when this process of glycosylation malfunctions, the risk of developing diseases such as cancer, diabetes, Alzheimer’s, and muscular dystrophy significantly increases. Researchers have recently made strides in understanding how the interaction between a key enzyme and a small structure in glycans can contribute to these breakdowns.
In the field of glycobiology, the enzymes responsible for glycan production in humans, known as glycotransferases, have been extensively studied and categorized. However, there is still a need for a deeper understanding of how these enzymes are regulated within cells to predict and fine-tune glycan structures accurately. Researchers have focused on two types of glycans, N-linked glycans attached to nitrogen atoms within proteins, and O-linked glycans attached to oxygen atoms within proteins, to uncover the regulatory mechanisms of glycotransferases.
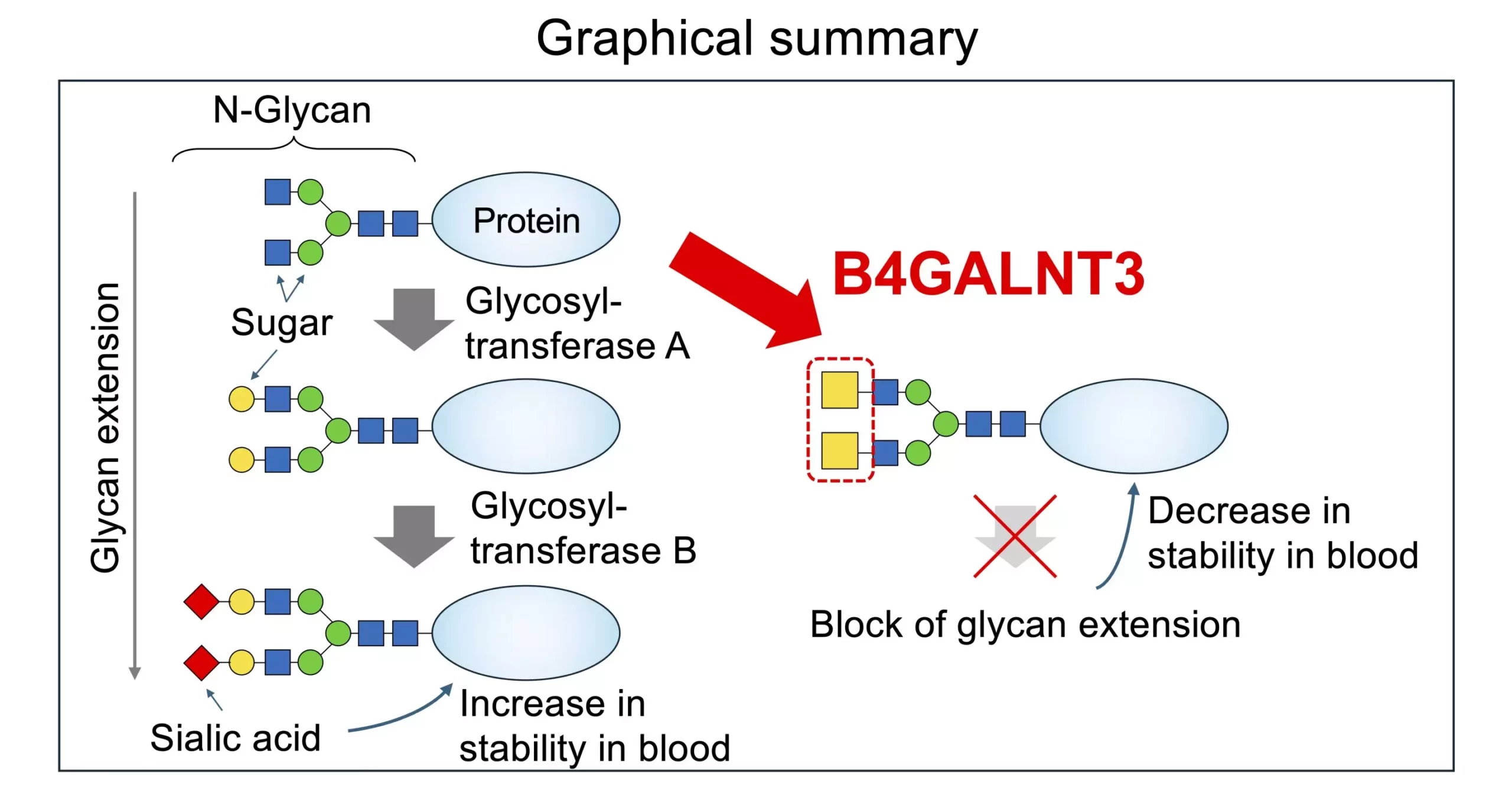
Within these glycans, a two-sugar structure called LacdiNAc, or LDN, has been identified at the terminal or sub-terminal positions. The biosynthesis of LDN is catalyzed by enzymes such as B4GALNT3 or B4GALNT4. The presence of LDN in glycans has been associated with important bodily processes such as hormone modification and stem cell maintenance. Variants of the enzyme B4GALNT3 have also been linked to conditions like lower bone density, fractures, and increased cancer risk, highlighting the significance of understanding LDN biosynthesis.
Recent advances in structural biology and glycomics have provided researchers with new tools to investigate the synthesis of LDN. By analyzing the structures of human glycosyltransferases, researchers discovered a unique feature, the “PA14 domain,” in B4GALNT enzymes. Through mutational studies and biochemical analyses, they found that the PA14 domain is crucial for the enzymatic activity of B4GALNT3 in N-linked and O-linked glycans as well as glycoproteins. Additionally, the presence of LDN significantly impacts glycosyltransferase activity in modifying N-glycans, influencing the structure and function of glycoproteins.
Understanding the role of B4GALNT3 and LDN in glycan structures is essential for unravelling disease pathogenesis and identifying potential therapeutic targets. By delving deeper into the regulatory mechanisms of glycotransferases and the impact of LDN on glycan structures, researchers aim to develop targeted interventions for diseases associated with glycosylation malfunctions. The study of glycans and their interactions with enzymes opens up new avenues for precision medicine and personalized treatments for a range of diseases.
Natural gas leaks are a growing concern in both urban and rural settings, with potential…
Recent groundbreaking research at the University of Vienna has unveiled a novel interplay of forces…
In recent years, perovskites have garnered significant attention in the fields of materials science and…
For decades, astronomers have probed the depths of the Milky Way, grappling with two perplexing…
Foreign direct investment (FDI) in developing nations has long been heralded as a path to…
As spring beckons in April and May, stargazers have the unique opportunity to witness nature's…
This website uses cookies.