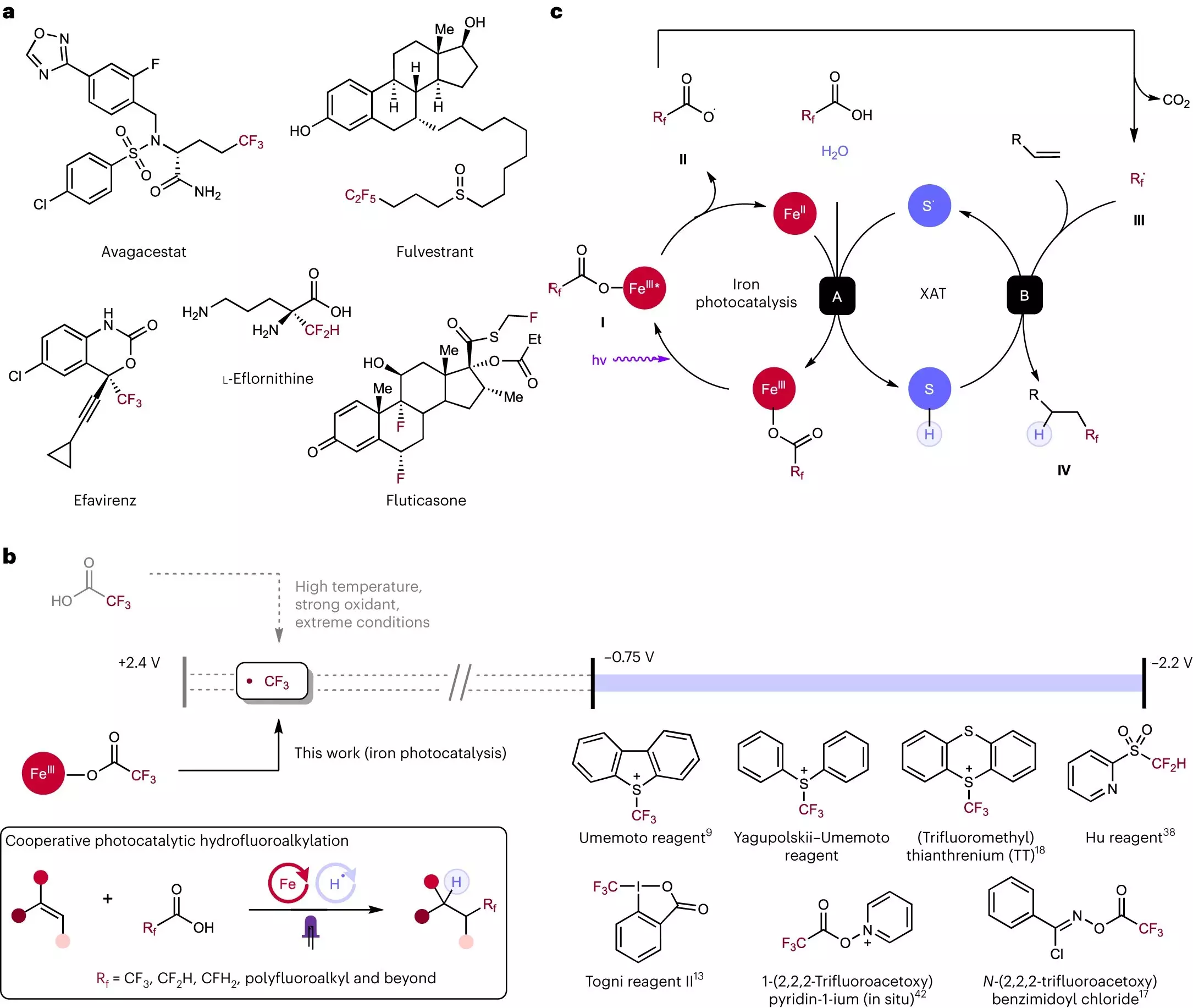
Chemical reactions play a critical role in drug development, and one atom that has gained a reputation as a “magic bullet” in this process is fluorine. Fluorine has the unique ability to increase a drug’s absorption and prolong its lifetime, making it a highly sought-after component in pharmaceuticals. However, traditional methods of incorporating fluorine into compounds have been expensive and challenging. Recognizing this problem, scientists at Rice University have developed a groundbreaking process that utilizes an iron and sulfur reaction to add fluorine to molecules. This innovative technique, as described in a study published in Nature Chemistry, not only simplifies and streamlines the fluorination process but also significantly reduces costs.
Before delving into the details of the novel process, it is essential to understand the significance of fluorine in drug development. Fluorine has been found to enhance the action and efficacy of numerous pharmaceuticals, including well-known medications such as Prozac and Lipitor. When attached to drug molecules, fluorine enables treatments to act faster and for longer durations, while also minimizing potential side effects. However, the challenge lies in efficiently incorporating fluorine during the drug manufacturing process. Existing methods often rely on expensive and highly reactive chemicals that are not always effective. With this in mind, the researchers at Rice University embarked on a mission to discover a safe, cost-effective alternative.
At the forefront of this groundbreaking research is Julian West, the Norman Hackerman-Welch Young Investigator and assistant professor of chemistry at Rice University. Driven by a “pie-in-the-sky” idea, West’s research group aimed to find a way to add fluorine to molecules through a cheap, safe, and efficient process. Their journey began by exploring the potential of iron and sulfur, which are readily available and inexpensive elements. Previous studies had already demonstrated that iron and sulfur could combine to perform decarboxylation reactions, during which carboxylic acid molecules could be broken down, releasing fluorine atoms.
Carboxylic acids, which are abundant and stable compounds, posed a challenge due to their resistance to traditional methods of modification. However, West’s team discovered that when subjected to light, iron and sulfur interact and disrupt the stability of carboxylic acids, liberating fluorine atoms for further use. With the fluorine atoms now available, the researchers had to determine how to reattach them to molecules effectively and safely.
Rice University doctoral alum Yen-Chu Lu and chemistry graduate student Kang-Jie (Harry) Bian played a pivotal role in solving this puzzle. They found that fluorinated carboxylic acids could react with alkenes, which are commonly found in drug molecules. Alkenes serve as versatile building blocks, allowing for the creation of more complex drug components. The same iron and sulfur combination that facilitated the decarboxylation of carboxylic acids proved instrumental in attaching the fluorine fragments to alkenes.
Not only is the newly developed strategy fast, simple, and safe, but it also offers a wide range of potential applications. By utilizing different carboxylic acids, researchers can introduce various fluorine components into molecules, broadening the possibilities in drug development. To validate the reliability of the process, Rice graduate student Shih-Chieh Kao and undergraduates Xiaowei Chen and David Nemoto Jr. conducted rigorous testing and confirmed the efficacy of the technique. Further exploration is underway, as researchers continue to push the boundaries of this revolutionary approach.
The process of fluorinating molecules for enhanced pharmaceutical efficiency has received a significant boost with the discovery of a light-activated iron and sulfur reaction. This breakthrough, pioneered by scientists at Rice University, opens up new avenues for easier, cheaper, and more effective fluorination in the drug development field. With the ability to add fluorine to molecules in a safe, efficient, and cost-effective manner, researchers are now equipped with a powerful tool to enhance the efficacy of pharmaceutical drugs and potentially minimize side effects.
In the world of pharmaceuticals, innovation often hinges on finding new compounds that can lead…
In the heart of the Amazon basin, drastic climate changes present an alarming reality that…
Air fryers have rapidly surged in popularity, captivating home cooks and culinary enthusiasts alike. When…
In an era where technology and social media reign, the importance of sleep often takes…
In an era where environmental consciousness is paramount, the maritime industry has long been scrutinized…
Radionuclides, often relegated to discussions surrounding nuclear energy and radioactive waste, have far-ranging implications for…
This website uses cookies.