Organic synthesis, the process of creating molecules used in various industries, is like playing with microscopic LEGO bricks. Chemists connect simple building blocks to form complex molecules, just as LEGO bricks are snapped together to construct intricate structures. A crucial step in this process involves creating a bond between two carbon atoms. However, connecting these carbon atoms can be challenging because the most reactive carbon atoms carry a positive charge, which makes them incompatible. This article explores the concept of organometallic compounds and their significance in organic synthesis, as well as the rediscovery of an abandoned method called the Barbier method using mechanochemistry.
In the nineteenth century, researchers discovered that by bonding carbon to metals like zinc or magnesium, they could switch the charge of carbon atoms from positive to negative. This “polarity switch” enabled the creation of suitable combinations with other organic molecules, expanding the possibilities in organic synthesis. Victor Grignard, a French chemist, made one of the most influential discoveries in this field by inventing a method for creating organic derivatives of magnesium. His technique, known as the Grignard method, revolutionized organic synthesis and earned him a Nobel Prize in 1912.
However, the Grignard method had its limitations. The metal-containing molecules produced were highly reactive and prone to breaking down when exposed to moisture or air, making large-scale applications challenging. Researchers needed to find a solution that would allow the generation of more stable compounds.
Philip Barbier, Grignard’s scientific teacher, attempted to achieve carbon atom bonding using a similar method but encountered unsatisfactory results. The yields of the desired products were low, and the technique fell by the wayside. Interestingly, he assigned the task of perfecting his method to Grignard, who went on to make the Nobel-winning discovery. Despite being a pioneer of organometallic chemistry, Philip Barbier did not receive the same recognition.
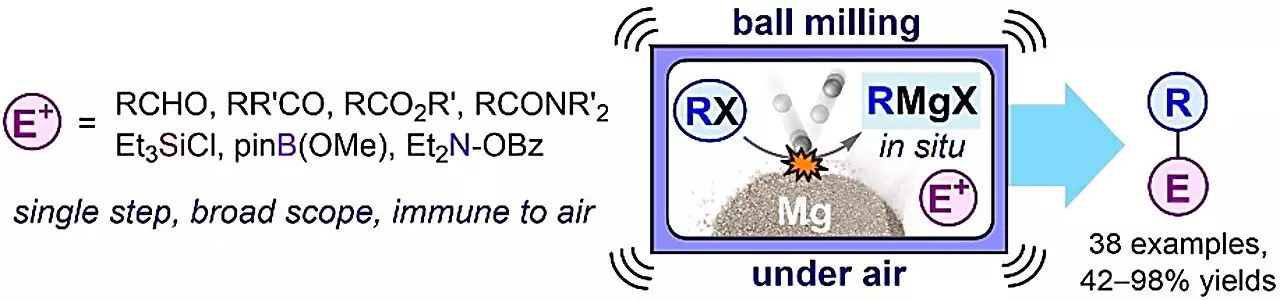
Recently, a group of chemists from the supramolecular chemistry research group of TalTech led by Prof. Riina Aav and Dr. Dzmitry Kananovich breathed new life into the previously abandoned Barbier method. Instead of using organic solvents and mixing chemicals with magnesium metal, the researchers discovered that milling them together without a solvent in a shaker mill was highly effective and environmentally friendly. This innovative approach has rejuvenated the Barbier method, making it as efficient as the renowned Grignard method. The findings of this study were published in Angewandte Chemie International Edition.
Mechanochemistry, the technique employed by the TalTech researchers, involves chemical reactions occurring through quick blending, milling, and grinding of solid substances. This ancient technique had been largely ignored by the scientific community in favor of solution-based chemistry. However, its benefits for the environment and safety standards have reignited interest.
Mechanochemistry eliminates the need for dangerous organic solvents, which pose risks to both human health and the environment. This aspect makes it particularly appealing for the preparation of organometallic compounds, an area of significant focus in chemistry. The TalTech research team revisited Barbier’s original idea, simplifying the use of organometallic compounds. This new method also exhibits resistance to air and weak acids, which is advantageous over traditional approaches like the Grignard technique. Since the organometallic compounds exist only as intermediates, they can continue reacting and create stable end products, promising a revolution in the production of valuable substances.
The rediscovery and advancement of mechanochemistry could have a profound impact on the chemical industry, paving the way for safer and more sustainable processes. It has the potential to revolutionize the way substances are manufactured, leading to simpler and more environmentally friendly methods. Industries, such as pharmaceuticals, with significant impacts on society, can benefit from this innovation. The TalTech research team, in collaboration with researchers from eleven European countries, is working on the IMPACTIVE project to further develop mechanochemical production methods and transform the pharmaceutical sector.
The rediscovery of the Barbier method through mechanochemistry offers a glimpse into the future of organic synthesis. This blend of old and new techniques presents exciting opportunities for the chemical industry. By harnessing the benefits of mechanochemistry, scientists can create safer, more sustainable processes that will benefit future generations. The potential applications of this innovative approach extend beyond pharmaceuticals, making it a promising field for future research and development.
Rogue waves have long been a subject of fascination and terror in maritime lore. These…
As the world grapples with public health challenges, especially those posed by infectious diseases, the…
The Sombrero Galaxy, also known as Messier 104, embodies a breathtaking blend of spirals and…
In recent advances in quantum electronics, a groundbreaking discovery leveraging the concept of kink states…
In the intricate tapestry of nature, ice often exists in a delicate balance with liquid…
In an astonishing event that captured global attention, a rogue object from beyond our Solar…
This website uses cookies.