Photocatalytic hydrogen evolution from water is a crucial technology for sustainable hydrogen production, yet the impact of interfacial water molecules on photocatalytic reactivity is not well understood. A recent study published in the Journal of the American Chemical Society sheds light on the roles of interfacial hydrogen bond structure and dynamics in promoting H2 evolution. The researchers demonstrated the importance of the optimal interfacial water environment for enhancing photocatalytic performance.
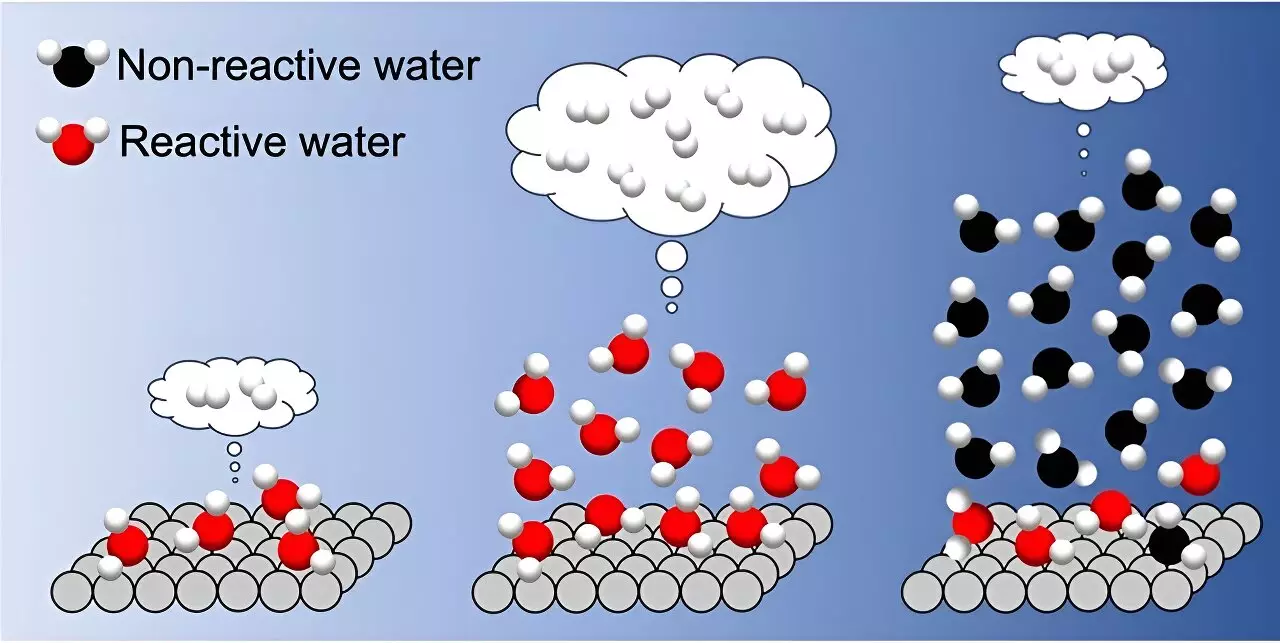
The study, led by researchers at the Institute for Molecular Science, explored the interfacial hydrogen bond networks using various TiO2 photocatalysts. By controlling the thickness of adsorbed water layers, the researchers identified a direct correlation between H2 formation rate and the microscopic structure of H-bond networks. They found that as water adsorption increased up to three layers, the H2 formation rate also increased linearly. However, when more than three layers of water covered the TiO2 surface, the H2 formation rate drastically decreased.
The researchers observed two distinct types of adsorbed water on the TiO2 surface: interfacial water and liquid-like water. The presence of liquid-like water in more than three layers led to strengthening of the interfacial H-bond, hindering interfacial proton-coupled hole transfer and reducing the H2 formation rate. Based on these findings, the researchers suggest that depositing three water layers in a water vapor environment is optimal for photocatalytic hydrogen evolution.
Traditionally, photocatalysis has been studied in aqueous solution environments, but this study represents a potential paradigm shift by demonstrating the effectiveness of water vapor environments. These results open up new possibilities for the design and engineering of interfacial water in photocatalytic systems for renewable energy production.
The study highlights the crucial roles of interfacial hydrogen bond structure and dynamics in promoting photocatalytic hydrogen evolution. By understanding the impact of interfacial water molecules on reactivity, researchers can design innovative photocatalysts for sustainable hydrogen production. The findings of this study provide valuable insights for the development of next-generation energy technologies.
In an era where environmental consciousness is paramount, the maritime industry has long been scrutinized…
Radionuclides, often relegated to discussions surrounding nuclear energy and radioactive waste, have far-ranging implications for…
Landslides have long been a concern in areas like California, where the unique geography and…
In the vastness of our galaxy, among countless stars, lies a fascinating phenomenon known as…
This week marks a monumental milestone in astronomy as the Hubble Space Telescope celebrates its…
The enigma of dark matter has captivated the scientific community for decades. Although it constitutes…
This website uses cookies.