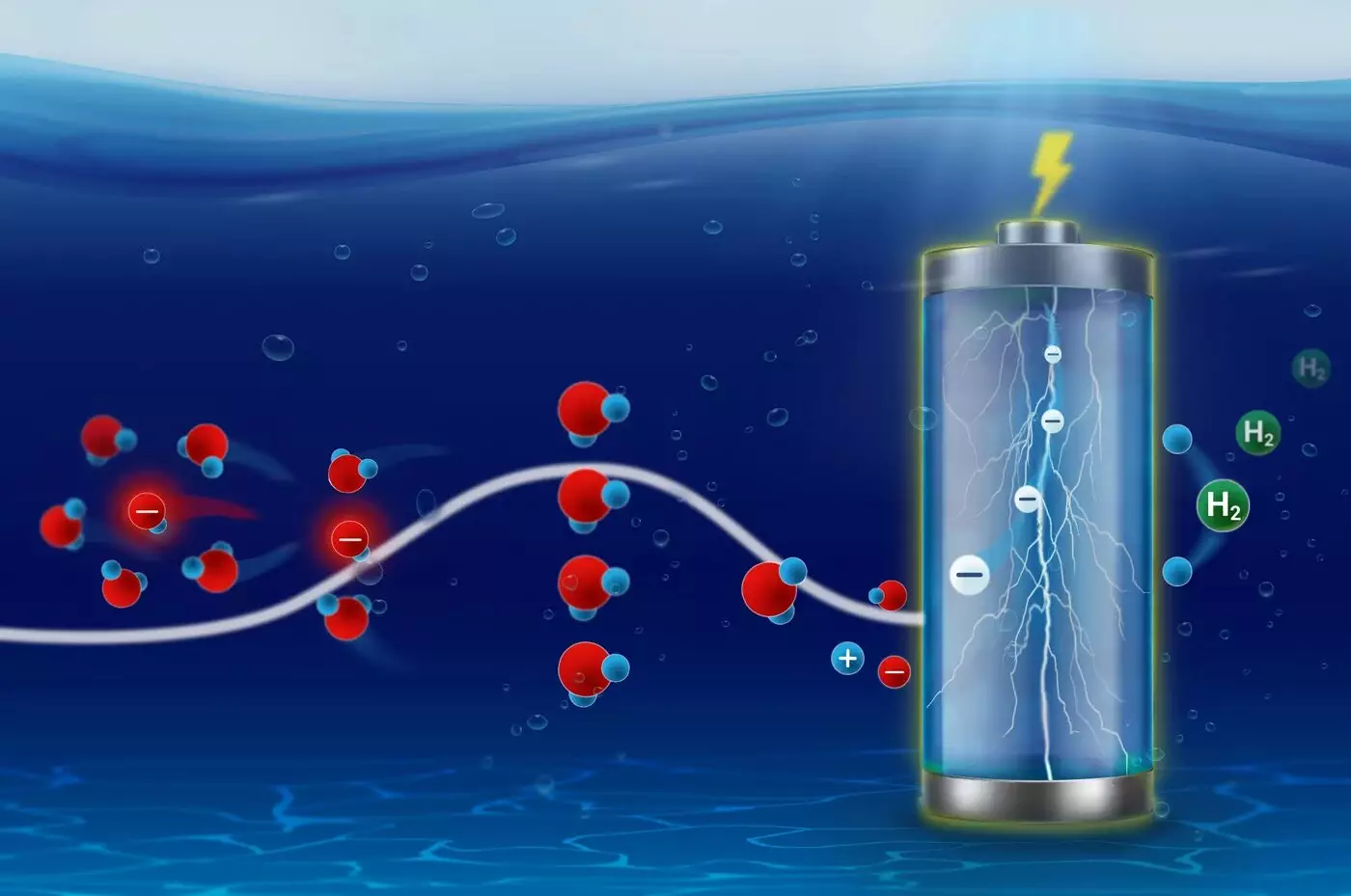
The behavior of ions in various electrochemical processes is fundamental to advancements in energy storage and conversion technologies. Whether in batteries, bioelectrochemical systems, or electrocatalysts, the ability of ions to reorganize their solvation shells determines their effectiveness and efficiency in these applications. Recent insights from researchers at the Fritz Haber Institute’s Interface Science Department shed light on the critical role that solvation kinetics play in these electrochemical phenomena.
The act of intercalation into battery cathodes or the absorption and conversion processes on electrocatalytic surfaces is far from straightforward. Ions must negotiate their way through their solvation environments, and the manner in which they do this can dictate the overall performance of the system. Traditionally viewed through a deterministic lens, this complex ion behavior is influenced by variations in activation entropy and enthalpy, an understanding that has been brought to light through pioneering research interpreting ion kinetics via statistical physics.
The research team employed the Eyring-Evans-Polanyi equation, a cornerstone of transition state theory formulated nearly a century ago. The study’s primary author, Francisco Sarabia, elaborates on the innovation of measuring activation energy parameters with millisecond resolution. With this advancement, researchers have a clearer view of the electrosorption kinetics of hydroxide ions, which are known to interact with specific structural motifs on surfaces.
This innovative approach highlights how step-edges and defects within a catalyst surface can significantly affect ion behavior. Specifically, the researchers have revealed new dynamics regarding the poisoning behavior of platinum (Pt) surfaces during ammonia oxidation reactions. This finding marks a significant leap forward, providing insights that were previously obscured by traditional observation methods.
The consequences of these findings extend beyond practical applications; they challenge existing paradigms within the field. Prevailing theories maintain that activation energy is the primary determinant in the dependency of electrocatalytic reactions on applied bias. However, this recent work suggests that activation entropy also plays a pivotal role, illustrating how changes in pH can induce non-Nernstian behaviors. This revelation encourages a deeper investigation into the electrochemical environment surrounding catalysts and the intricate link between ionic behavior and catalytic efficiency.
Dr. Sebastian Öner, a leading figure in the Interface Science Department, emphasizes the wealth of operando evidence supporting the notion that catalyst surfaces are highly dynamic despite prior assumptions. This dynamicity entails that the interaction between solid structures and liquid electrolytes cannot be overstated; rather, they must be viewed as co-dependent entities that influence each other’s properties and behaviors.
This comprehensive understanding of solvation kinetics opens up new avenues for the development of more efficient catalysts. By acknowledging the interplay between the solid and liquid phases in electrochemical reactions, researchers can design catalysts that not only exhibit improved activity but also selected stability across varied operating conditions. The Interface Science Department, under the leadership of Prof. Dr. Beatriz Roldán Cuenya, is poised to delve deeper into these insights, with the potential for transformative impacts in energy and chemical conversion applications.
The evolving comprehension of ion dynamics and solvation kinetics presents a pivotal turn in electrochemical research. It encourages researchers to reconsider long-held assumptions and lays a new groundwork for future exploration in enhancing the efficacy of electrocatalysts and energy systems. The path ahead promises to be as intricate as the ion movements that drive these essential processes, ultimately aiming for a more sustainable and efficient technological landscape.
In the realm of software development, the ability to swiftly and accurately address bugs is…
The realm of quantum computing and communication is not just an abstract dream anymore; it…
In a remarkable leap for the field of material science, a collaborative research initiative has…
Throughout Earth's vast history, our planet has endured five major mass extinction events that reshaped…
Rainfall is a vital element of our planet’s hydrological cycle, yet many aspects of its…
On a night when the universe aligns, a mesmerizing phenomenon awaits: the appearance of the…
This website uses cookies.