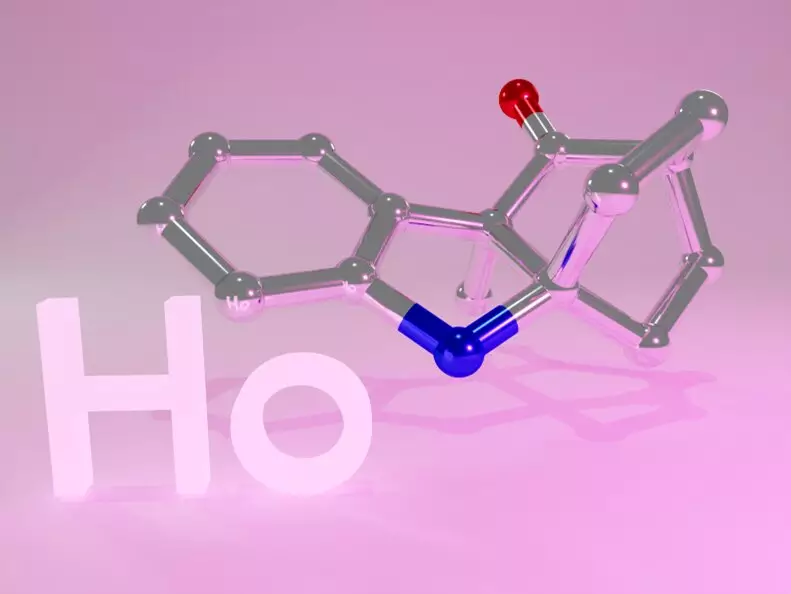
Hydrocarbazole is a key compound in organic chemistry, playing a crucial role as a building block for various biologically active compounds. These compounds include pesticides like strychnine and anticancer drugs such as vinblastine and minovincine. Given the importance of hydrocarbazole in the synthesis of such compounds, the development of efficient synthesis methods is a significant research area in the field of organic chemistry.
Associate Professor Shinji Harada and his research team from Japan have been at the forefront of research in this field. Their recent breakthrough involved the use of organic compounds known as indole-incorporated siloxydienes along with rare earth catalysts. While their progress has been noteworthy, there is still room for improvement in terms of the generality of substrates and catalyst reactivity.
One of the key challenges in synthesizing hydrocarbazole compounds is the low reactivity of siloxydiene substrates, particularly those with substituents at the second carbon position of the indole ring. These substrates are essential for creating hydrocarbazole compounds with tetrasubstituted carbon, such as the compound Kopsinine, which has potential applications in pharmacological research.
To tackle this issue, Dr. Harada and his team, along with Professor Miki Hasegawa, developed a new technique for synthesizing complex hydrocarbazole compounds with tetrasubstituted carbon. This method utilizes a new lanthanide-based catalyst that offers high purity in compound synthesis. Additionally, the catalyst can be recycled, making the process more sustainable and environmentally friendly.
Initially, the researchers experimented with a chiral helical ytterbium catalyst but faced challenges due to the low reactivity of the substrate. To enhance the catalyst’s performance, they modified it by incorporating triflimide salt, resulting in a ytterbium triflimide catalyst. Subsequent modifications, such as replacing the central metal with holmium, led to a significant improvement in both yield and enantioselectivity of the synthesized hydrocarbazole compounds.
The developed technique showed versatility in synthesizing multiple complex hydrocarbazole compounds, including a tetracyclic compound with five chiral centers. The researchers also delved into studying the reaction mechanisms through experimental and computational methods. Dr. Harada emphasized the broader impact of the research, stating that the findings could accelerate the development of new drugs and positively influence various aspects of human life, from medicine to the environment and food.
The research led by Dr. Shinji Harada and his team represents a significant advancement in the synthesis methods for hydrocarbazole compounds. By addressing the challenges related to substrate reactivity and catalyst efficiency, the developed technique offers a promising approach to synthesizing complex compounds with high purity and sustainability. The potential applications of this research extend beyond the realm of organic chemistry, impacting various sectors and contributing to the improvement of global health and quality of life.
A groundbreaking expedition led by an international research team, featuring esteemed scientists from the University…
The pursuit of coherent control over wave transport and localization stands as a monumental challenge…
In recent astronomical explorations, researchers have unearthed a striking phenomenon emanating from a distant corner…
The quest for sustainable practices within the chemical industry is more critical than ever. Researchers…
In the complex interplay of human health, the relationship between the gut and the brain…
The relentless drive for sustainable energy solutions has fueled remarkable advancements in solar technology, with…
This website uses cookies.