Peptides, which are short strands of amino acids, have been gaining popularity for various applications such as therapeutics, biomaterials, and chemical probes. One key challenge in working with peptides is the ability to attach functional molecules, like fluorescent compounds, to specific locations on the peptide without affecting its structure and function. The N-terminus of a peptide, which is the end with a free amine group (NH2), is an ideal location for adding functional molecules as it minimizes interference.
Attaching functional molecules to the N-terminus of peptides has been a challenge due to various limitations. Earlier methods often resulted in the release of functional groups in physiological conditions, allowed only one functional group attachment at a time, lacked uniformity in attaching molecules, or simply had inefficient reactions. These limitations hindered the progress in peptide modifications for various applications.
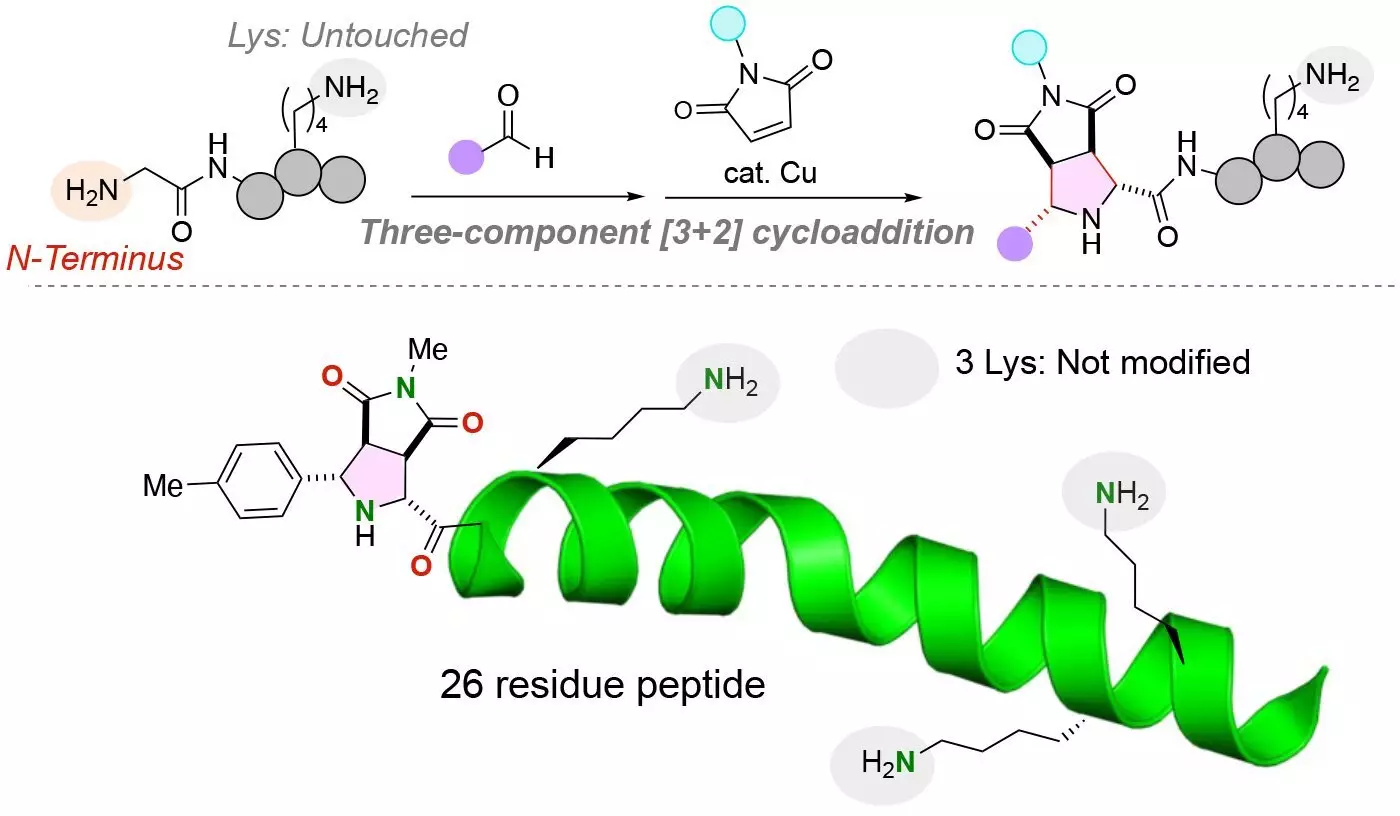
Recent research by teams from Tohoku University and Chuo University led to a significant breakthrough in the site-selective dual modification of peptides. They developed a unique chemical reaction that allows the attachment of two distinct functional molecules to the N-terminus of a peptide containing a glycine amino acid. This innovative approach overcomes the challenges posed by reactive lysine residues and ensures structurally uniform conjugates with high yields.
The research, published in the journal Angewandte Chemie International Edition, highlights the capability of exclusively functionalizing the N-terminus of peptides, regardless of the presence of lysine residues. By using a copper catalyst in a three-component reaction involving peptides, aldehydes, and maleimides, the team successfully attached the functional molecules in a single pot under mild conditions. This resulted in stable carbon-carbon bonds between the N-terminus of the peptide and the functional molecules.
One of the key challenges in peptide modification has been the presence of lysine amino acids, which contain amine groups that could interfere with the modification process. The developed chemical reaction specifically targets and labels the N-terminus amine group of peptides, even in the presence of lysine amino acids with alternative amine groups. This breakthrough simplifies the addition of functional molecules to peptides, opening up possibilities for diverse peptide labeling applications.
The research team has demonstrated the versatility of the dual modification reaction by optimizing it for di-, tri-, and oligopeptides, showcasing its potential for labeling various peptides and larger proteins. Ongoing studies involve testing the biological activity of modified peptides and exploring the extension of the protocol to proteins and antibodies. These advancements hold promise for enhancing drug delivery systems and other biomedical applications.
The innovative site-selective dual modification of peptides opens up new possibilities for the field of peptide research and applications. By overcoming previous limitations and addressing challenges posed by reactive amino acids, researchers are paving the way for the development of novel peptide-based therapeutics, biomaterials, and diagnostic tools. The continued exploration of this dual modification protocol is expected to lead to further advancements in peptide chemistry and contribute to the broader field of biopharmaceuticals.
A groundbreaking discovery by scientists at the University of Manchester is poised to reshape the…
In an era marked by rapid ecological change, the quest for understanding environmental pollutants like…
The meteoric rise of artificial intelligence (AI) technologies is not without significant implications on our…
The landscape of quantum computing is on the verge of transformative progress, fueled by groundbreaking…
Schizophrenia is not merely a mental health issue; it is a multifaceted condition that wreaks…
Recent scientific research has illuminated the intricate web that ties our dietary choices to the…
This website uses cookies.