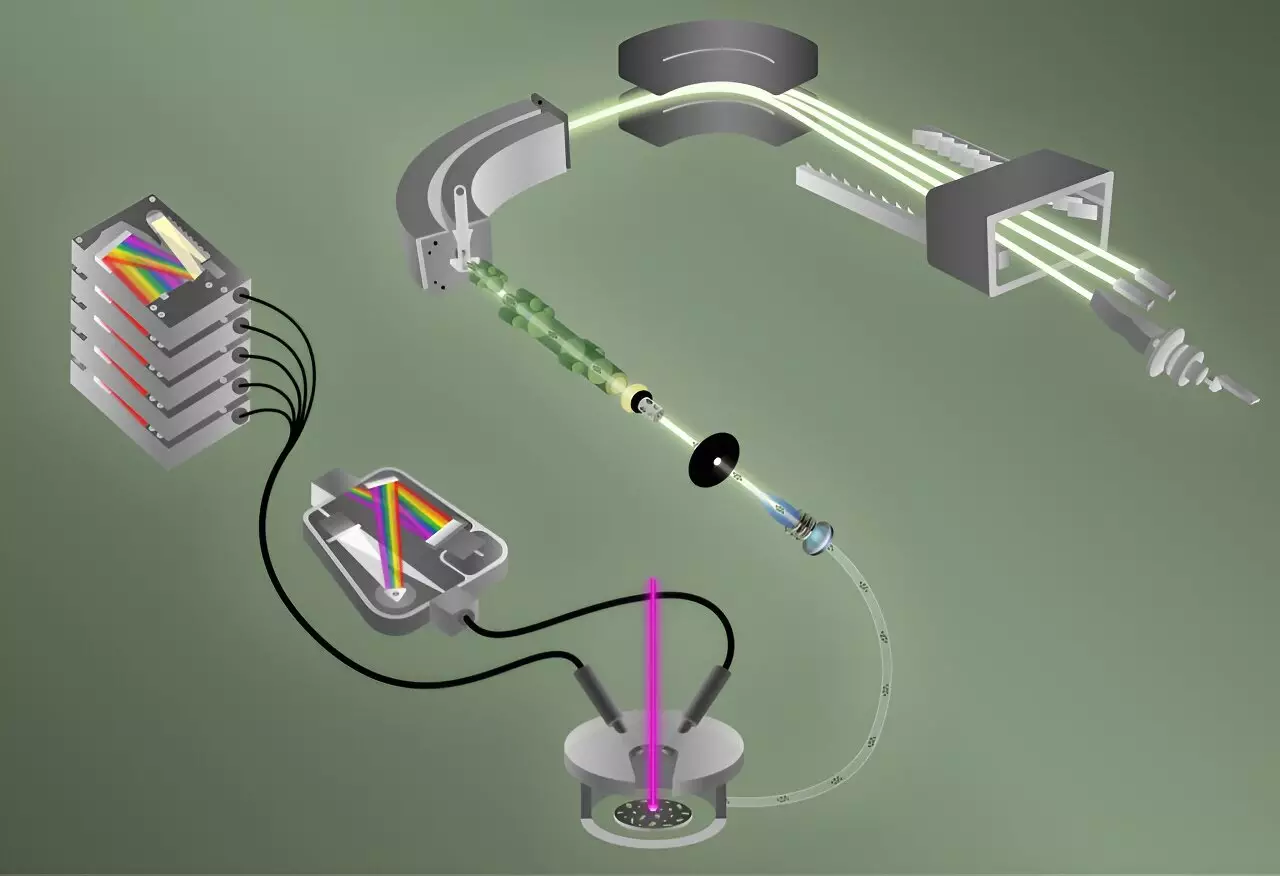
Advancements in analytical chemistry often pave the way for significant strides in a multitude of sectors, particularly in national security related to nuclear materials. Researchers at the Oak Ridge National Laboratory (ORNL), part of the U.S. Department of Energy, have achieved a remarkable feat: for the first time, they have successfully detected both fluorine and various isotopes of uranium within a single particle simultaneously. This innovative technique carries implications that may enhance the effectiveness of inspectors from the International Atomic Energy Agency (IAEA) by providing crucial insights into the application of nuclear materials. The study, recently published in the Journal of the American Chemical Society, represents a significant advancement in particle characterization, pushing the boundaries of speed and accuracy in chemical analysis.
At the heart of this achievement is the integration of two sophisticated analytical methods: Laser-Induced Breakdown Spectroscopy (LIBS) and Laser Ablation Multicollector Inductively Coupled Plasma Mass Spectrometry (ICP-MS). This collaborative approach has allowed researchers to analyze 40 particles, each roughly the size of a red blood cell, in less than five minutes—an achievement that significantly outpaces traditional methods that could take hours or even days.
LIBS functions by rapidly vaporizing the sample, creating a plasma that emits light as it cools down, analogous to fireworks that illuminate different wavelengths corresponding to different elements. This technique is particularly adept at identifying elemental fluorine due to its high sensitivity. Simultaneously, the ICP-MS technique—known for its ability to characterize isotopic compositions at extreme temperatures (as hot as 8,000 kelvin)—captures the isotopes of uranium present in the same particle.
According to Benjamin Manard, the lead researcher for this initiative, integrating these two techniques allows for immediate and accurate analytics regarding the presence of both fluorine and uranium isotopes within a single particle, streamlining what was previously a labor-intensive process.
The detection of fluorine alongside uranium holds substantial meaning, particularly from a nuclear nonproliferation viewpoint. As fluorine plays a vital role in converting uranium into a form that is suitable for enrichment processes, the ability to ascertain the ratio of these two elements can yield valuable information about the origins and processes that produced the particle. Co-author Brian Ticknor emphasized that analyzing the presence of fluorine in conjunction with uranium could provide insights into the historical timelines of nuclear material production.
Moreover, the implications of this research extend beyond national security. The techniques developed through this collaborative effort could find applications in various fields, including advanced battery manufacturing, studies of environmental contamination, and investigations into the transport of microplastics. The potential versatility of these analytical methods signifies a broader impact that could enhance scientific understanding across multiple disciplines.
Despite the innovative nature of these combined techniques, there are challenges inherent in the integration. As Ticknor explained, traditional mass spectrometry methods require the target elements to form positive ions for successful analysis. However, fluorine’s tendency to hold a negative charge complicates its detection through conventional ICP-MS. This necessitates the dual approach of using LIBS to analyze fluorine and ICP-MS for uranium isotopes, filling a gap that had not previously been addressed in a cohesive manner.
Efforts to meld these methods were made possible through the collaborative work of a multidisciplinary team at ORNL’s Ultra-trace Forensic Science Center. Each member contributed their expertise, ultimately refining a technique that not only enhances analytical speed but also improves precision.
Looking ahead, this ongoing research harbors promising possibilities for future investigations. The ability to distinguish various uranium compounds could greatly enhance nuclear material analysis. Interestingly, researchers are also considering extending their dual-technique analysis to other challenging elements such as chlorine. As both fluorine and chlorine exhibit electronegative characteristics, developing optimized strategies for their detection could reveal even more about the complexities of nuclear processes.
Moreover, ORNL researchers have begun to apply their findings to fields outside nuclear science. For example, they have utilized the LIBS to map fluorine distribution in shark teeth, providing a unique glimpse into environmental conditions over time. This highlights the adaptive nature of their research and its potential relevance in both basic science and applied fields.
The pioneering integration of LIBS and ICP-MS at Oak Ridge National Laboratory marks a significant leap in the field of analytical chemistry, illuminating pathways for future explorations in nuclear material characterization and beyond. This development not only enhances our understanding of nuclear processes but also presents exciting opportunities for interdisciplinary applications. As researchers continue to refine and expand upon these techniques, we can anticipate a richer comprehension of both current and historical contexts relevant to nuclear materials and their broader implications in science and technology.
Despite the term "rare," rare earth metals (REMs) are not nearly as scarce as their…
A collaboration led by Rutgers University-New Brunswick has initiated a paradigm shift in our understanding…
Natural gas leaks are a growing concern in both urban and rural settings, with potential…
Recent groundbreaking research at the University of Vienna has unveiled a novel interplay of forces…
In recent years, perovskites have garnered significant attention in the fields of materials science and…
For decades, astronomers have probed the depths of the Milky Way, grappling with two perplexing…
This website uses cookies.