In a world increasingly defined by climate change and resource depletion, the need for innovative solutions to traditional energy problems has never been more urgent. One such breakthrough is emerging from the fusion of chemistry and electrical engineering, where scientists are actively working to convert waste carbon emissions into viable energy sources. Recent advancements in this arena—especially those detailed in a groundbreaking study published in *Nature Catalysis*—shed light on converting carbon dioxide into methanol, a promising liquid fuel. This article delves into the intricacies of this innovative process and its potential implications for our energy landscape.
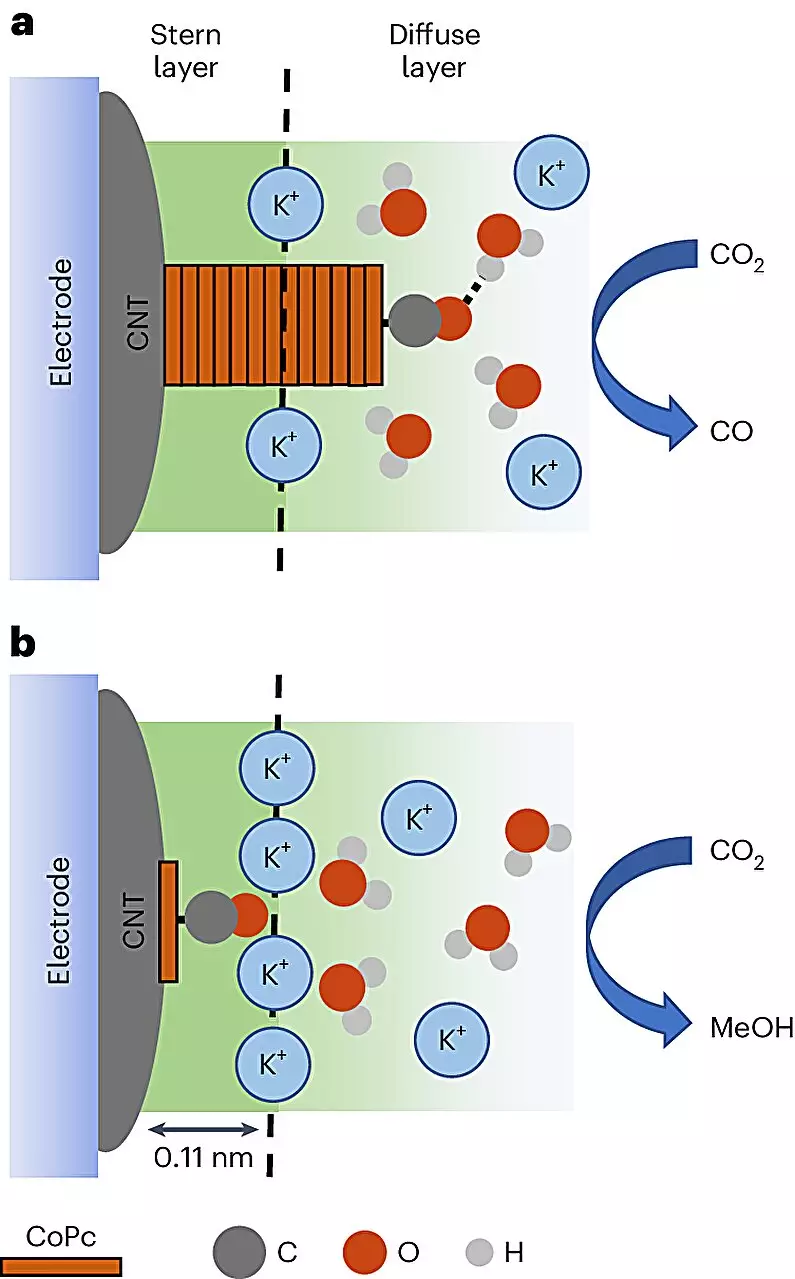
At the heart of this study lies a sophisticated process developed through international collaboration. Researchers employed cobalt phthalocyanine (CoPc) molecules, which serve as a catalyst, and supported them on the fascinating structure of carbon nanotubes—conductive materials with remarkable electrical properties. The methodological brilliance of this approach is rooted in the strategic application of an electrolyte solution, activated by an electric current. This creates the necessary conditions for CoPc molecules to engage in a chemical dance, effectively utilizing electrons to convert carbon dioxide into methanol.
Remarkably, the study showcased the first-ever visual observations of this transformation. Using cutting-edge in-situ spectroscopy, the researchers gleaned valuable insights into the molecular behavior during the reaction, revealing a critical finding: the reaction’s outcome—methanol versus carbon monoxide—hinges on the surrounding environment where the carbon dioxide is reacted. This environmental tuning opened the door for increased efficiency, with methanol production swelling by as much as eightfold under optimal conditions. Such revelations bear the promise of revolutionizing other catalytic processes across various scientific domains.
Methanol is not merely a chemical curiosity; it stands as a beacon of potential in the quest for sustainable energy solutions. Its high energy density makes it an appealing candidate for an alternative fuel source, suitable for vehicles ranging from cars to airplanes. Robert Baker, a co-author of the study and a prominent figure in chemistry and biochemistry, elaborates on methanol’s desirability: “When you take carbon dioxide and convert it to another product, there are many different molecules you can make. Methanol is definitely one of the most desirable.”
The implications of this research extend beyond just fuel. Methanol can play a vital role in heating, power generation, and even act as a stepping stone for future chemical innovations. The potential uses of a clean, renewable form of methanol fuel signify a substantial leap towards energy independence while addressing the grave ecological challenge of climate change.
While the empirical optimization of chemical reactions has been a common practice, comprehending the exact mechanisms behind these processes has often eluded researchers. Baker astutely points out the challenges, noting the difficulty in determining why certain catalysts outperform others. This knowledge gap can stymie progress toward more effective energy solutions. However, the current study’s revelations mark a pivotal step in bridging this divide.
Scientists like Quansong Zhu, the lead author on the project, employed sophisticated vibrational spectroscopy techniques to unveil how molecules behave on surfaces, making it possible to identify the conditions leading to optimal methanol production. The researchers discovered that specific cations—highly charged particles—enhanced the methanol formation process. As this field advances, understanding these cation dynamics could further refine the method, leading to more efficient production techniques.
The study represents a formidable leap forward, yet it is merely the starting point. As scientists tease apart the complexities of this reaction and related processes, we stand at the precipice of a new era in energy production. The promising data points toward broader applications, but it also raises critical questions: What other substances can we synthesize from carbon emissions? How can we scale up this approach to meet global energy demands sustainably?
Baker envisions a future where the knowledge gained from this research could initiate a cascade of innovations—”There’s a lot of exciting things that can come next based on what we’ve learned here.” As we march forward into an era punctuated by climate challenges, initiatives like this serve as a clarion call to embrace scientific ingenuity, transforming potential threats into genuine opportunities for a greener and more sustainable future.
Water contamination by nitrates poses a dire threat not only to ecosystems but also to…
When we think of objects entering water, the classic image conjured is of a drop…
Alzheimer’s disease is often thought of as a condition that primarily affects the elderly, yet…
As the Earth's temperature escalates, the Arctic tundra stands at a critical threshold, impacting global…
One of the most mesmerizing predictions stemming from Einstein's theory of general relativity is the…
In a remarkable development that could reshape the landscape of communication for those with speech…
This website uses cookies.