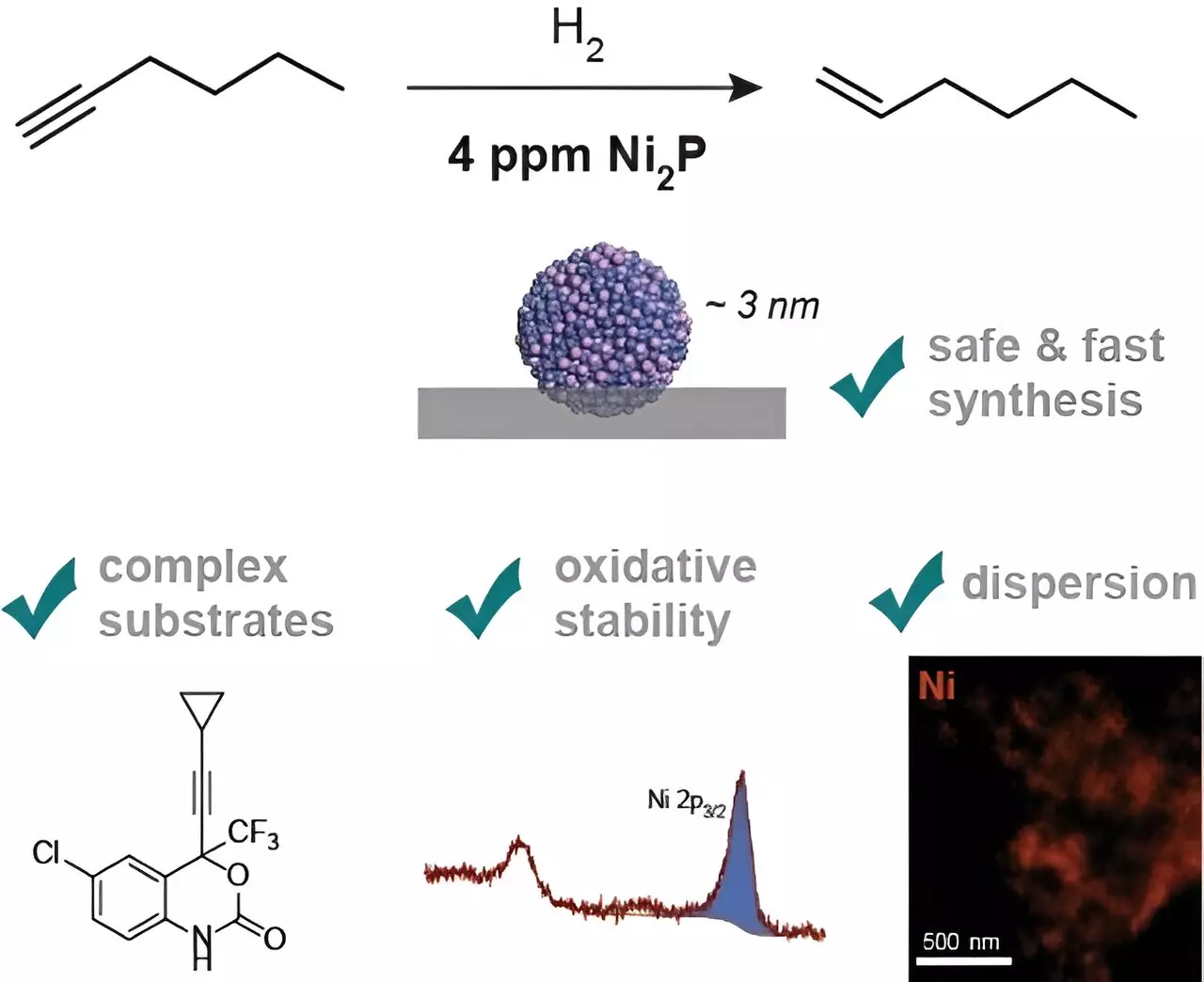
Transition metal phosphides have long been considered a promising alternative to noble metal catalysts due to their cost-effectiveness and abundance. However, challenges such as surface oxidation and complex synthesis processes have hindered their widespread adoption. Dr. Constanze Neumann, a leading researcher at the Max-Planck-Institut für Kohlenforschung, and her team have made significant strides in addressing these challenges.
Dr. Neumann and her team have developed a novel single-step synthesis method using safe and inexpensive materials to create an air-stable, nickel-containing catalyst. By employing the right surface ligands, the researchers were able to achieve a high dispersion of the catalyst on a carrier surface, ensuring optimal distribution for the desired reaction. This breakthrough not only improves the efficiency of the catalyst but also allows it to be used in smaller quantities compared to conventional palladium catalysts.
The new catalyst developed by Dr. Neumann’s team has been shown to be on par with commercial palladium catalysts, making it an attractive option for chemical companies, especially in the synthesis of pharmaceuticals. What sets this catalyst apart is its remarkable stability even after prolonged exposure to air. Unlike other phosphides that require specialized handling in a glovebox, the Mülheim catalyst can be easily managed in a standard fume hood, simplifying storage and handling processes.
Despite their success, Dr. Neumann and her team are not resting on their laurels. They aim to enhance the reusability of their catalyst and eliminate the need for solvents in its production. By continuously pushing the boundaries of catalyst innovation, they are paving the way for a more sustainable and efficient future in chemical catalysis.
The groundbreaking work of Dr. Neumann and her team in developing transition metal phosphide catalysts represents a significant advancement in the field of catalysis. By addressing key challenges, improving efficiency, and ensuring long-term stability, they have set a new standard for catalyst development. As they continue to explore new possibilities and innovations, the impact of their research is poised to revolutionize the way we approach chemical synthesis and catalysis.
In the realm of software development, the ability to swiftly and accurately address bugs is…
The realm of quantum computing and communication is not just an abstract dream anymore; it…
In a remarkable leap for the field of material science, a collaborative research initiative has…
Throughout Earth's vast history, our planet has endured five major mass extinction events that reshaped…
Rainfall is a vital element of our planet’s hydrological cycle, yet many aspects of its…
On a night when the universe aligns, a mesmerizing phenomenon awaits: the appearance of the…
This website uses cookies.