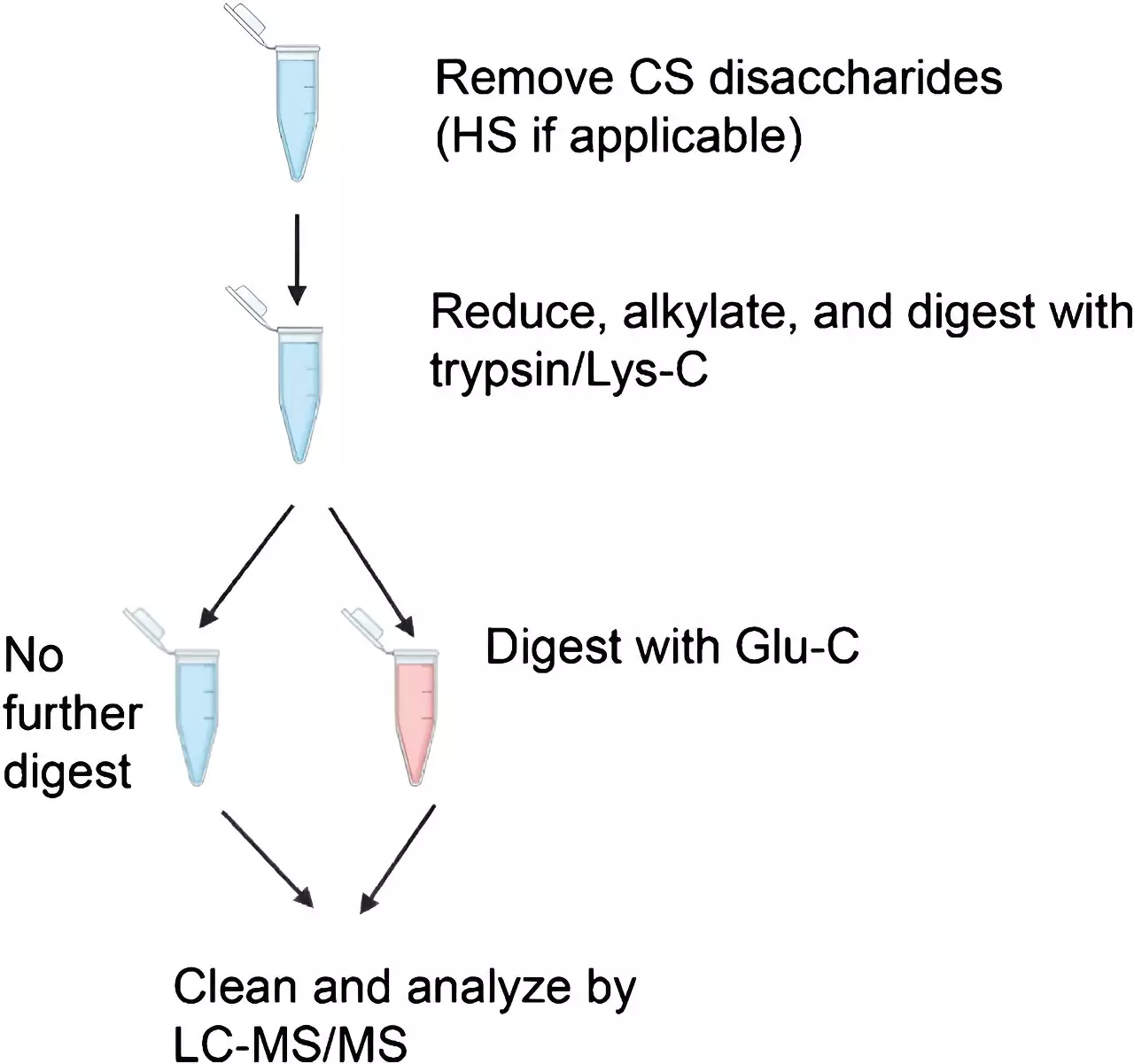
One of the fundamental processes in molecular biology is glycosylation, which involves the attachment of carbohydrates to protein molecules. This enzymatic reaction is crucial in determining the structure, function, and stability of proteins. Proteins that undergo glycosylation are referred to as glycoproteins. The two main types of protein glycosylation are N-glycosylation and O-glycosylation.
Researchers at Boston University Chobanian & Avedisian School of Medicine recently conducted a study with the aim of improving the methods used to identify glycopeptides. The study found that conventional mass spectrometry methods were effective in identifying peptides with one glycan, but additional steps were required to identify peptides with two or more glycans. This is significant because a single protein can carry multiple glycans, making it challenging to generate peptides that only carry one glycan. Identifying peptides with multiple glycans is essential in understanding the various glycoforms of proteins and their role in biological function and disease.
Despite the importance of glycoproteins in disease development and progression, there is still a lack of comprehensive knowledge about their behavior and how they change during various stages of development and disease. Manveen K. Sethi, the corresponding author of the study, believes that this lack of understanding may lead to missed therapeutic opportunities. By gaining a better understanding of how glycosylation affects diseases, particularly site-specific alterations, new targets for therapeutic intervention could be identified. Sethi emphasizes that advances in technology that enable the confident identification of densely glycosylated peptides would be instrumental in differentiating healthy and diseased states based on site-specific glycosylation. This, in turn, would allow for the use of glycoproteins as both markers and therapeutic targets in various diseases.
The researchers utilized four different standard proteins and employed various enzymatic digestion protocols prior to conducting mass spectrometry analysis. They compared two mass spectrometry methods: conventional higher-energy collisional dissociation (HCD) and the more recently developed electron transfer/higher energy collisional dissociation (EThcD). The objective was to determine the efficacy of each method in identifying glycopeptides. The study revealed that while HCD is sufficient for glycopeptide identification, EThcD is often necessary to detect glycans, especially in the case of multiply-glycosylated peptides.
The research conducted at Boston University sheds light on the importance of glycosylation in protein structure and function. By improving methods to identify and analyze glycopeptides, scientists can unravel the complexities of glycosylation and its impact on diseases. This comprehensive understanding may lead to the identification of new therapeutic targets and the development of more effective treatment options for diseases that are currently lacking satisfactory remedies. The future of glycosylation research is promising, and continued advancements in technology will undoubtedly contribute to groundbreaking discoveries in the field of molecular biology.
In the realm of software development, the ability to swiftly and accurately address bugs is…
The realm of quantum computing and communication is not just an abstract dream anymore; it…
In a remarkable leap for the field of material science, a collaborative research initiative has…
Throughout Earth's vast history, our planet has endured five major mass extinction events that reshaped…
Rainfall is a vital element of our planet’s hydrological cycle, yet many aspects of its…
On a night when the universe aligns, a mesmerizing phenomenon awaits: the appearance of the…
This website uses cookies.