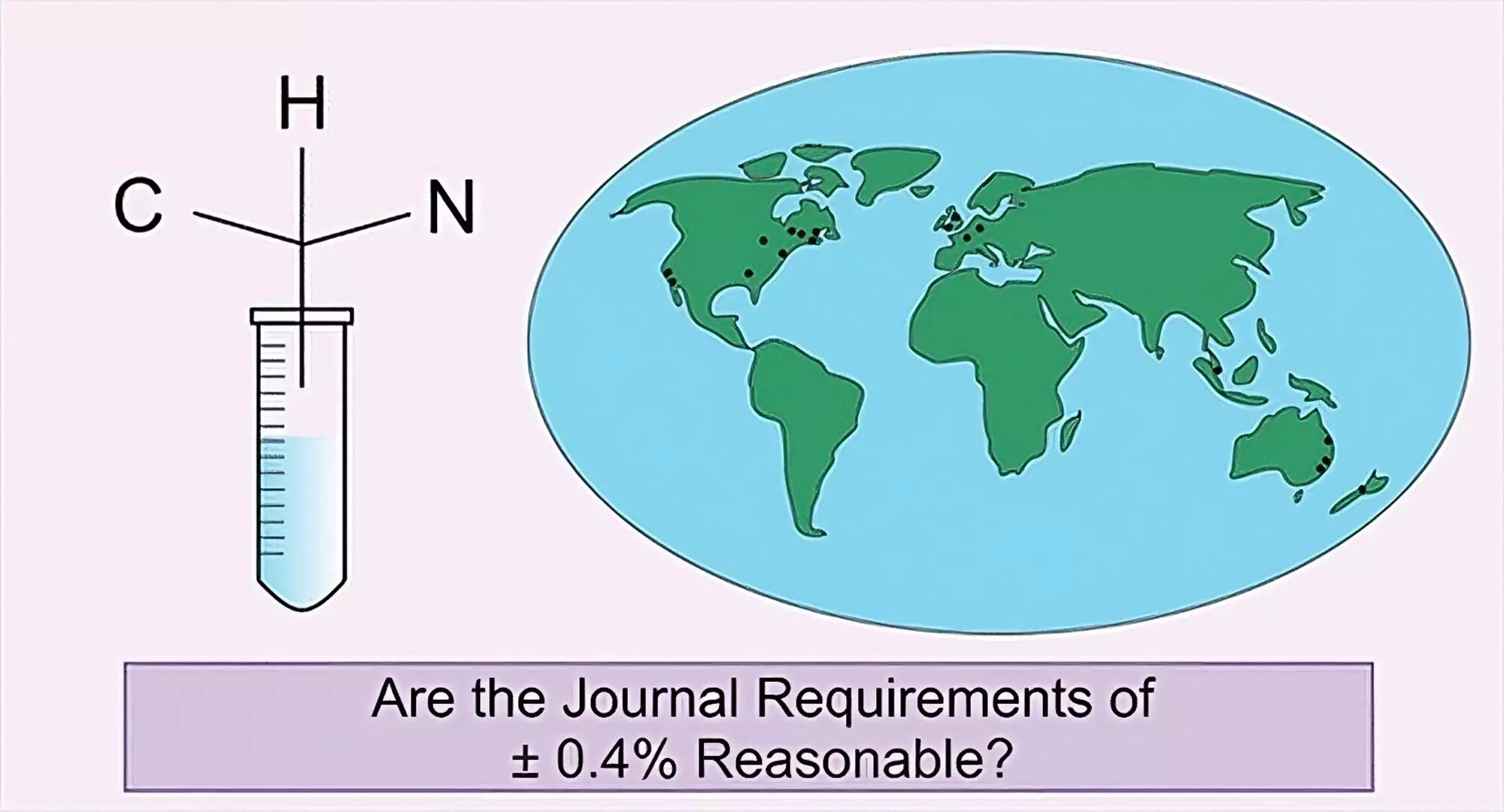
In 1923, Fritz Pregl was awarded the Nobel Prize in chemistry for his contributions to quantitative microanalysis, which became a crucial tool to determine the elements present in a sample or compound. This technique has been widely adopted in the field of chemistry to determine the identity and purity of a chemical. However, the long-accepted +/-0.4% standard for determining the value obtained may not always be accurate, depending on the nature of the compound, element assessed, and identity of the trace impurities. Therefore, an international research team led by a chemistry professor at Baylor University conducted the first-ever review of the validity of the standard.
Caleb Martin, Ph.D., associate professor of chemistry and biochemistry at Baylor University, conducted the study in 2022 to determine if the +/-0.4% standard is reasonable. The team’s study, titled “An International Study Evaluating Elemental Analysis,” was published in the journal ACS Central Science. In both of the two months following its publication in 2022, the study was the most read article in ASC Central Science and is still in the top 10 most-read list for the past 12 months. The study has sparked conversations among journal editors and chemists with an overall positive response.
The team sent identical samples of five organic compounds to 17 independent service providers from multiple countries and the in-house Chitnis Lab of team member, Saurabh S. Chitnis from Dalhousie University in Canada. Chitnis’s equipment was recently purchased and properly calibrated to ensure accuracy. The goal was to determine if there was deviation in measurement of each element beyond 0.4% in an identical set of compounds at facilities worldwide. A simple technique was used in which the compound is burned, and the combustion products are analyzed to determine its makeup by percentage of carbon, hydrogen, and nitrogen.
Each individual analysis obtained for carbon, hydrogen, and nitrogen was designated as a single data point. A data point was denoted “Fail” if it was not within 0.40% of the theoretical value and “Acceptable” if it was within 0.40% of the theoretical value as this is how scientific journals assess the data. The results from the service providers were compared with the established theoretical value and results from Chitnis’s equipment.
The results from the labs validated the team’s concerns on accuracy and reliability. In fact, 10.78% of the data points for the commercial samples tested did not meet publication guidelines despite being adequately pure. Graduate student Kanika Vashisth, who worked with Dr. Martin on the study, found the results unexpected. “It was quite surprising to obtain the failure results for commercially available compounds that are greater than 99.9% pure.”
Martin and his team concluded that the 0.4% purity standard is not a statistically realistic requirement for synthetic samples. Since their study, Chemistry-A European Journal and their sister journals under the Wiley publishing group have updated their guidelines to no longer include the 0.4% standard and have options when elemental analysis can’t be done. Other journals are also reevaluating their requirements with this article stemming editorial addresses to the community.
The team’s work has highlighted the need for more accurate and reliable analytical techniques in chemical research. Third-party companies are typically used to conduct the testing, and they are not required to provide corroborating raw data for the results, leaving researchers with little information on why the sample did not make the grade. Additionally, there is no oversight to ensure the machines used to determine purity are calibrated correctly, or accreditation mandated for staff. Errors translate into delays in completing research, unnecessary expenses, and experimentalists being incorrectly blamed for inadequately purifying samples.
Martin and his team acknowledge that there is more work to be done, but they are hopeful that their work will bring about positive change in the field of chemical research. The study has raised an essential question, which requires further exploration. The scientific community has responded positively to the study, and researchers are finding it quite an interesting study.
Rogue waves have long been a subject of fascination and terror in maritime lore. These…
As the world grapples with public health challenges, especially those posed by infectious diseases, the…
The Sombrero Galaxy, also known as Messier 104, embodies a breathtaking blend of spirals and…
In recent advances in quantum electronics, a groundbreaking discovery leveraging the concept of kink states…
In the intricate tapestry of nature, ice often exists in a delicate balance with liquid…
In an astonishing event that captured global attention, a rogue object from beyond our Solar…
This website uses cookies.